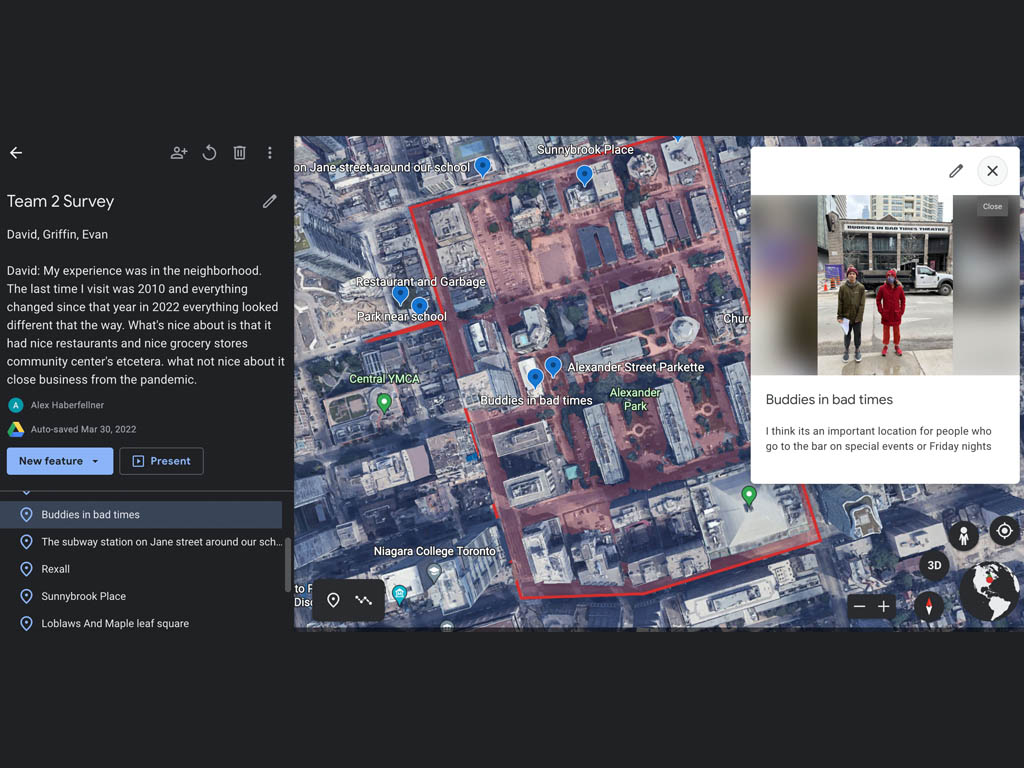
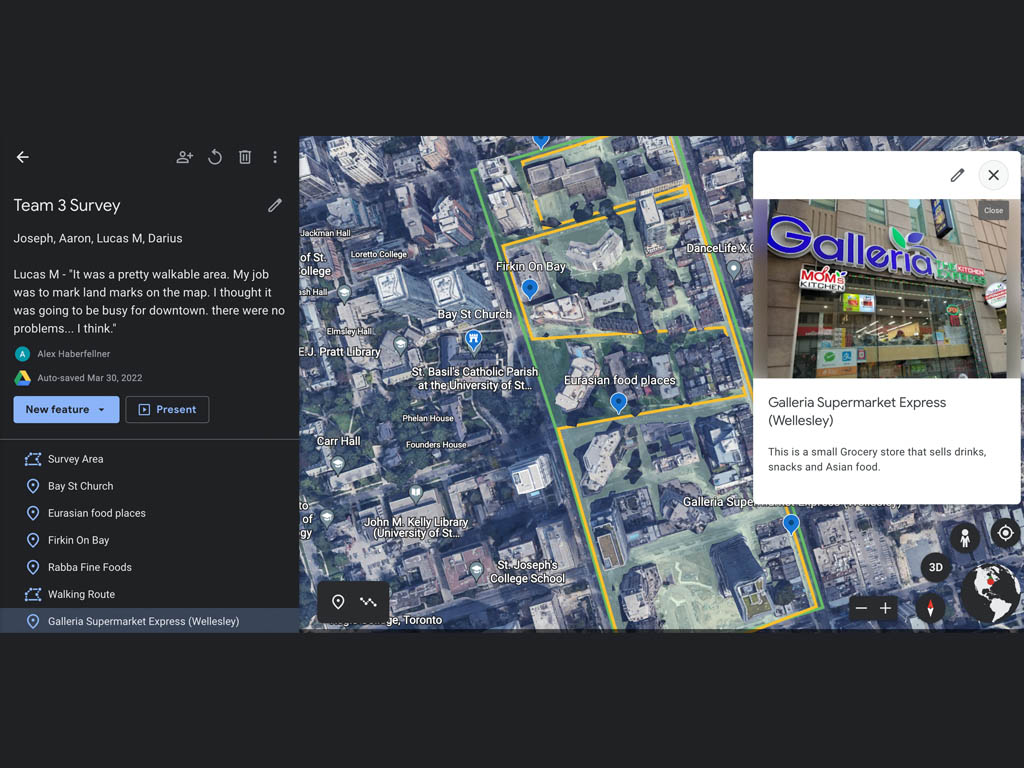
Early in the school year, the grade 10 science classes learned about quantitative and qualitative observations in the field. The groups headed out on a single-period walking excursion to Queen’s Park where they could conduct some observations in a dynamic environment. Students began by engaging their senses; feeling textures, smelling scents, observing colours, and listening to the sounds around them. Learning how terms like “lots,” “green,” “good,” and “cold,” represented judgements that could be considered qualitative was useful. Students also developed knowledge around how countable measurements of distance, weight, amount, temperature, volume, and area using standard units would be considered quantitative.
Students toured in small groups around the park, making both qualitative and quantitative observations. They were instructed to return to the whole group with three qualitative questions, and three quantitative questions that could be posed about features of the park. A group discussion was held about how that data could be gathered and verified.