There are six types of chemical reactions that are investigated in the grade ten chemistry unit: Synthesis, Decomposition, Single Displacement, Double Displacement, Combustion, and Acid-Base. Simply discussing and recognising the different types of chemical reactions can be somewhat uninteresting (Figure 1 – case in point) and even intimidating.
Figure 1
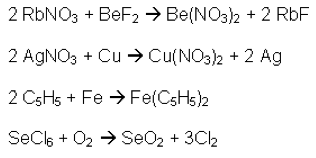
Life is not always about remembering equations and formulas, because let’s face it; most students forget the majority of the equations and formulas they have learned once they leave the walls of educational institutions. If, however, you can help students see through the equations and formulas and how they apply to the world they experience around them, they will remember that for many years after they have forgotten the formulas and equations.
In order to bring the chemistry from the whiteboard to life, we look at a real-time example of a decomposition reaction. We take a mixture of hydrogen peroxide and add to it a few drops of food colouring (for effect) and a few drops of liquid dish soap (for the wow factor). To that mixture we add a pre-mixed batch of warm water and yeast (to speed of the decomposition of the hydrogen peroxide). What ensues, and what the students witness and ooh and aah at is the oxygen produced from the decomposition of hydrogen peroxide quickly expanding the soap bubbles in the solution and quickly foaming up and over the graduated cylinder.
